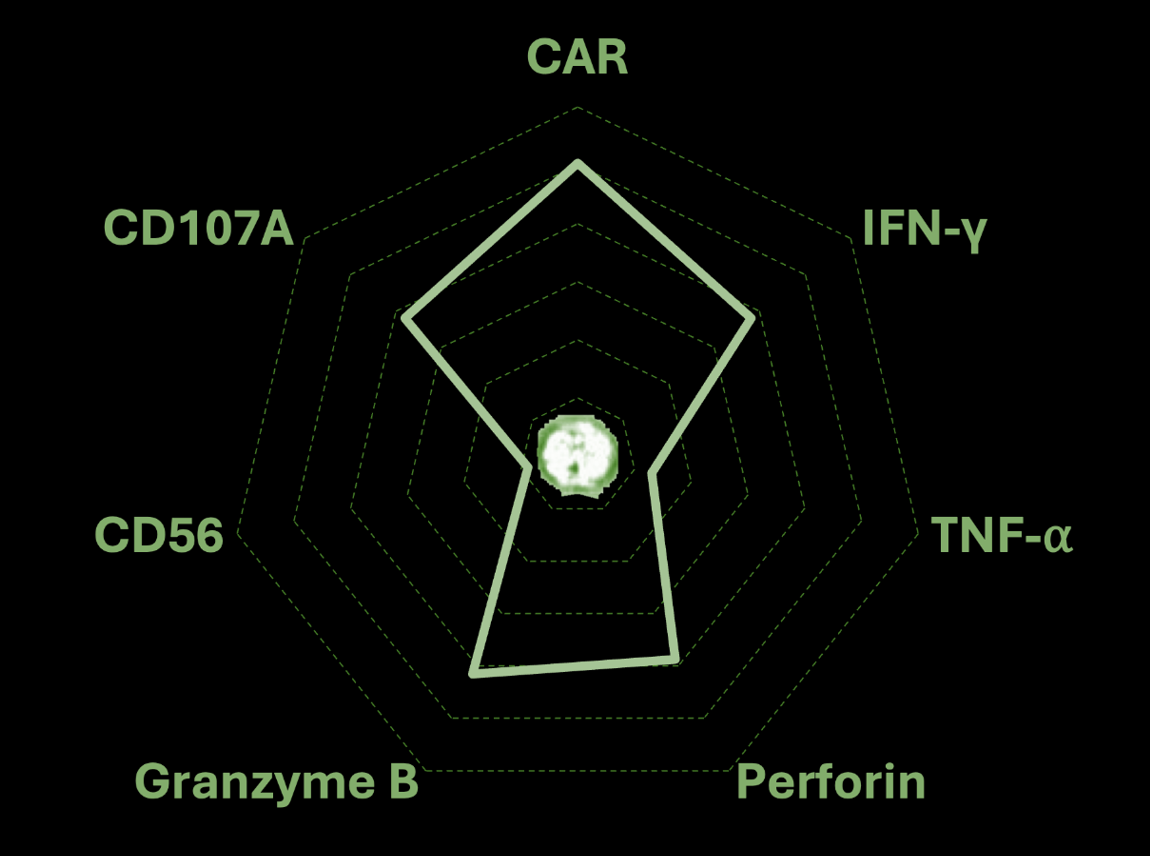
A blood-based assay for precise monitoring of persistence and function of therapeutic cells post infusion.
More sensitive than PCR/NGS
Provides full phenotypic view of every single therapeutic cell
Detects rare cells with sensitivity of 1 in 1,000,000
Learn more about how Scout™ can help your cell therapy program